FDA ROMS Application
- Victor Carlstrom
- Nov 1, 2025
- 2 min read
Project Type: Application Modernization
FDA's Regulatory Operations Management System (ROMS) was a large undertaking that involved not only modernizing systems but also combining multiple processes into a single application. This new application needed to accommodate multiple different stakeholder groups and be scalable to integrate more processes in the future. All of this was done utilizing Appian as the coding platform.
The core operations included in ROMS were:
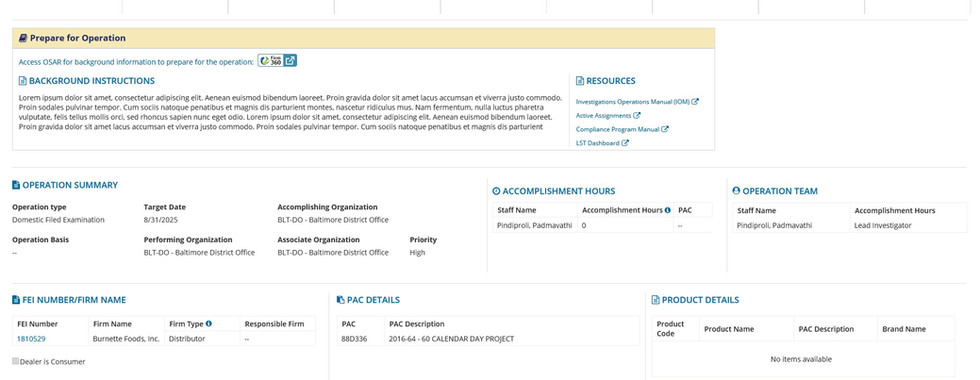
- Domestic Field Examinations: users would travel to a physical location to conduct an exam of the surroundings. Documentation of the exam would often include a report and possible further actions.
- Domestic Sample Collections: a team of users travel to a location to document any available products for regulatory purposes. This a labor and data intensive process that often took days to fully document, sometimes involving hundreds of subsamples collected at a time.
- Recall Audit Checks: should an organization be issued a recall, the FDA has protocols and procedures to follow in order to ensure compliance with instructions.

Human Centered Design
ROMS was designed together with FDA users to meet real-world needs from day one. Stakeholders participated in high-fidelity prototype testing, early feedback session, and scenario-based usability testing. We were able to go beyond normal user acceptance testing by introducing roadshows, one-on-one review, and real-time collaboration across multiple FDA offices. Our test scenarios were grounded in real business use cases, prompting more meaningful and actionable feedback.
Streamlined Workflows
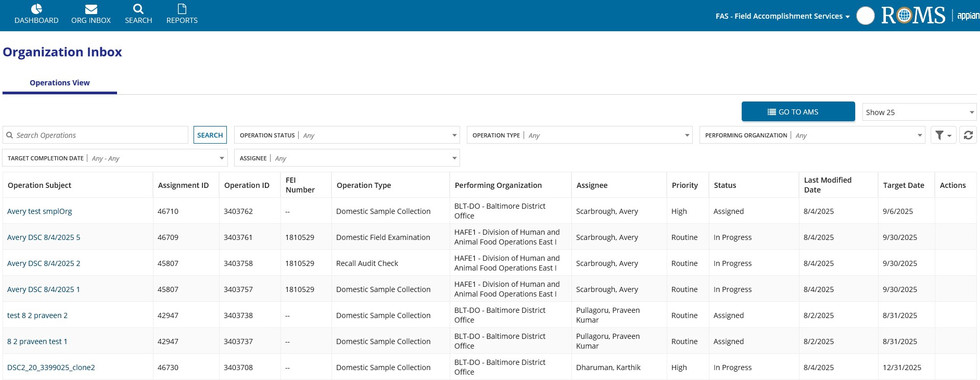
Remodeling the way the users approach their process gave us an opportunity to make meaningful impacts both within the application and outside of it. We established the Atomic Regulatory Process - a seven-step model that outlines the operations with all of their technical and logistical steps, their stakeholders at each stage, and how the application supports those actions. The functionality offered by the Appian platform allowed us to refactor the processes needed to achieve the customers' mission. Adjusting the process logic, dynamic form validation, and implementing automation created a system that is now saving countless hours of additional work for the FDA.
Operational Complexity
ROMS has a myriad of different stakeholders with different needs. Each of the operations incorporated needed their own analysis, user research, and solutioning to achieve success.
Enhanced Technical Value
Users reported a 183% increase in perceived technical value, reflecting how the new platform supports their work.
Increased User Satisfaction
Post launch surveys showed a 78% increase in user satisfaction and 69% improvement in system performance.


















Comments