ROMS UX Case Study
- Victor Carlstrom
- Dec 10, 2025
- 4 min read
Updated: Feb 1
Project Overview
The FDA's Regulatory Operations Management System (ROMS) represented a comprehensive modernization initiative that consolidated multiple critical regulatory processes into a single, scalable application. Built on the Appian low-code platform, this project aimed to unify disparate legacy systems while serving diverse stakeholder groups across the FDA's operational landscape.
The Scope: The modernization encompassed three primary operational areas:
Domestic Field Examinations: On-site facility inspections requiring comprehensive documentation and follow-up action protocols
Domestic Sample Collections: Complex, data-intensive processes involving the collection and documentation of hundreds of product subsamples over multiple days
Recall Audit Checks: Systematic compliance verification procedures following product recall issuances
The Vision: Create a unified platform that could accommodate multiple stakeholder groups with distinct workflows while maintaining scalability for future process integration and operational expansion.
My Role
Position: UI/UX Lead and Primary Designer
Key Responsibilities:
Led user experience strategy across multiple stakeholder groups
Designed comprehensive user research methodologies
Created design artifacts spanning from initial concepts to final prototypes
Collaborated with development teams in Agile sprint cycles
Leveraged previous FDA Appian project experience to inform platform constraints and opportunities
Strategic Contribution: Served as the bridge between complex regulatory requirements, diverse user needs, and technical platform limitations while ensuring design solutions met both usability and compliance standards.
Process and Methods
Comprehensive User Research Framework
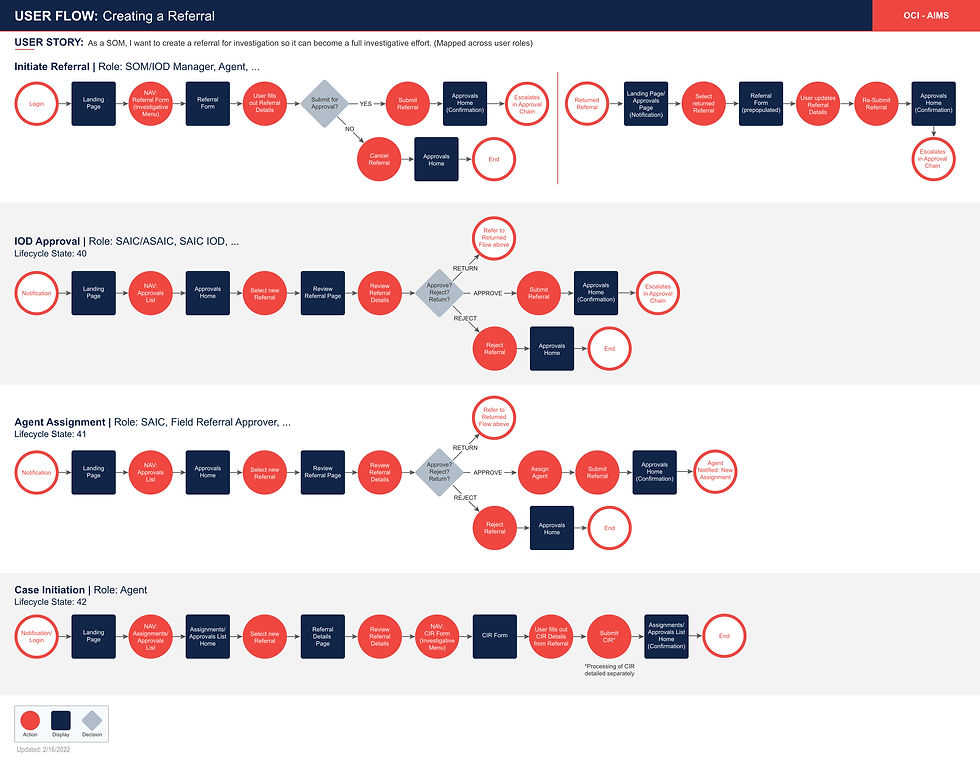
Atomic Process Development: Created detailed process maps encompassing the entire imports lifecycle, breaking down each operation into:
Pre-task preparation elements
Core operational activities
Post-task completion requirements
User roles and supervisory structures
Application touchpoints and data flows
Operational outcomes and downstream triggers
Research Methodology
Stakeholder Coordination: Organized knowledge sessions and structured interviews with each user group
Sequential Focus: Implemented rolling release strategy, concentrating on one operation at a time
Comprehensive Documentation: Developed complete user understanding through multiple research artifacts
Design Artifacts Created
Personas: Detailed user archetypes representing each stakeholder group
User Journeys: End-to-end experience maps across operational workflows
Task Flow Diagrams: Process visualization showing decision points and user paths
Wireframes/Mockups: Low and high-fidelity interface designs
Prototypes: Interactive representations for testing and validation
Usability Tests: Systematic evaluation of design effectiveness
User Surveys: Quantitative feedback collection and analysis
Agile Integration
Regular stakeholder review sessions for mockup validation
Continuous requirements gathering and refinement
Two-week sprint cycles with embedded design-development collaboration
Iterative design improvements based on ongoing feedback
Key Challenges
Multi-Stakeholder Complexity
Challenge: Each stakeholder group had distinct operational objectives, workflows, and success metrics, requiring tailored solutions within a unified platform.
Solution: Implemented sequential development approach, focusing on one operation at a time while identifying cross-group commonalities for solution reusability and consistency.
Legacy System Transition
Challenge: Users were deeply entrenched in outdated legacy applications, creating resistance to change and adoption barriers.
Solutions Implemented:
Modified Technology Acceptance Model (mTAM) Surveys: Targeted specific variables affecting user acceptance to inform design decisions
Comprehensive Usability Testing: Used clickable prototypes and scripted task sequences to gather authentic user feedback and identify improvement opportunities
Collaborative Training Development: Partnered with training teams to create resources explaining new processes, terminology, and functionality changes
Platform Constraints
Challenge: Appian's low-code platform imposed strict limitations on interface flexibility compared to custom-coded solutions.
Solution: Leveraged previous Appian experience to create a reusable Axure component library that mirrored platform capabilities while maintaining design quality and user experience standards.
Outcomes and Impacts
Quantitative Results
Pilot Program Success: Initial limited release with highly engaged users showed positive attitude shifts in 13 of 14 measured variables:
Technology Perception: +184% improvement
User Interface Satisfaction: +79% increase
Performance Rating: +69% enhancement
Operational Achievements
System Consolidation: Successfully replaced three separate legacy applications with single unified platform
User Base: Currently supporting 5,000+ active users
Transaction Volume: Processing 6,500+ inspection-related transactions monthly
User Satisfaction: Overall increase of 78% in user satisfaction scores
Process Improvements
Streamlined workflows across all associated regulatory processes
Enhanced workforce efficiency through consolidated operations
Improved data consistency and reporting capabilities
Reduced training overhead through unified interface design
Strategic Impact
The project's success has been recognized industry-wide, with detailed impact analysis available through REI Systems case study documentation.
Learnings
User Research Insights
Atomic Process Development: The comprehensive process mapping not only informed our design decisions but helped FDA stakeholders better understand their own operational systems, leading to identification and elimination of redundant features and workflows.
Survey Methodology Evolution: Significantly improved our approach to survey development, scoring methodologies, and results interpretation, creating repeatable frameworks for future projects.
Design Strategy Learnings
Platform Constraint Navigation: Developed effective strategies for creating compelling user experiences within low-code platform limitations, proving that thoughtful design can overcome technical restrictions.
Multi-Stakeholder Design: Refined approaches for balancing diverse user needs within unified platforms, creating scalable design patterns that accommodate various operational requirements.
Change Management Insights
Technology Acceptance: Demonstrated the effectiveness of research-driven change management, using quantitative measures to guide design decisions and improve adoption rates.
Training Integration: Established the critical importance of collaborative training development as part of the UX process, ensuring users understand both interface changes and underlying process improvements.
Scalability Validation
The project's success in accommodating future operational integration validates the scalable design approach, providing a framework for ongoing platform expansion and additional process consolidation.








Comments